A scientist in a race against time to find a cure for her daughter’s rare and deadly disease has said they are “finally in the driving seat”.
Dr Michelle Teng is trying to develop the world’s first treatment for TUBB4a leukodystrophy before it is too late for her 12-year-old daughter Sofia.
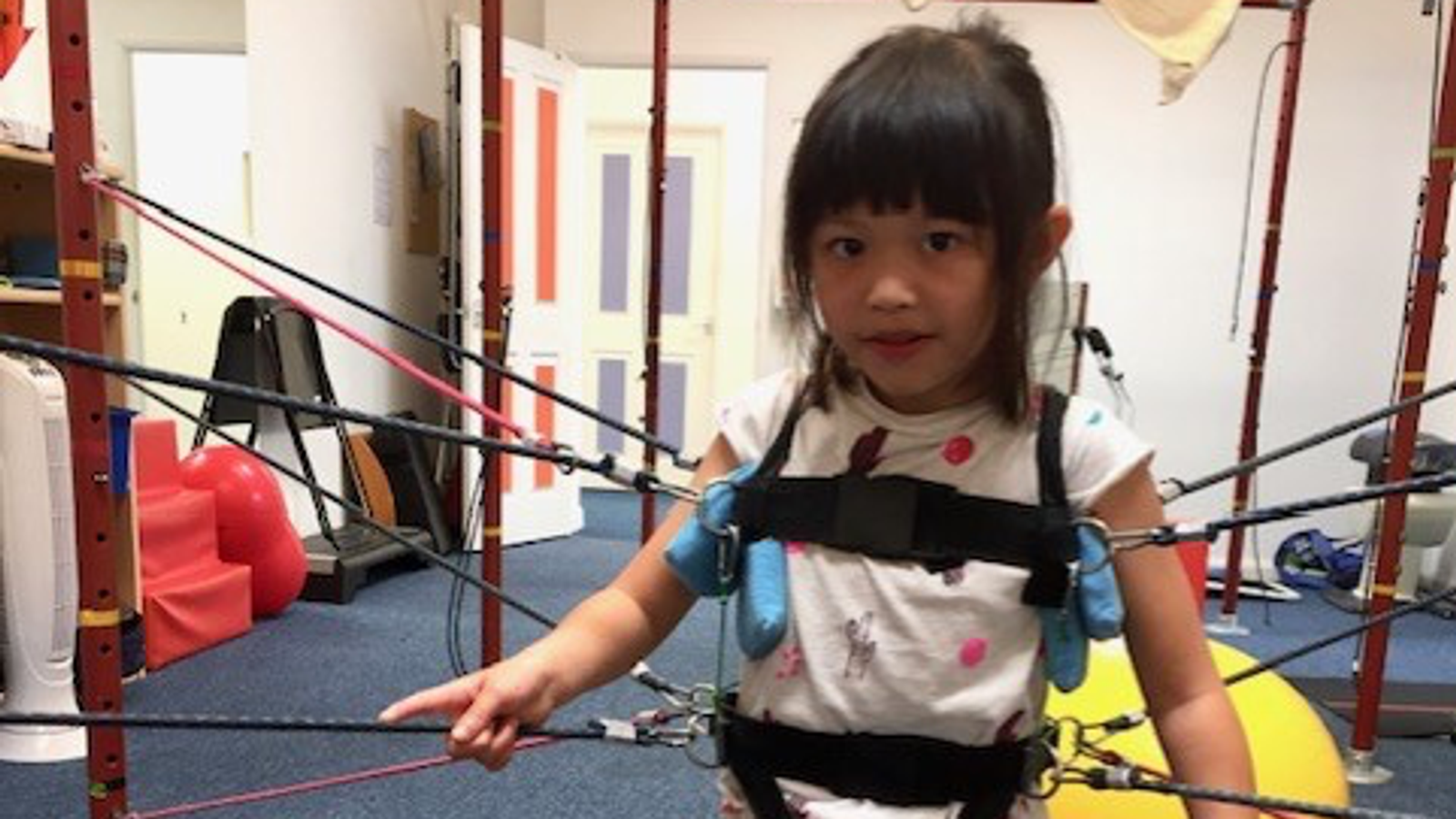
The life-threatening genetic condition affects the central nervous system and can lead to an inability to walk, talk and even swallow. Most young patients die before they reach adulthood.
Sofia started showing symptoms when she was a toddler and was eventually diagnosed with the disease aged four.
“When we Googled the condition, it was a terrifying experience,” Michelle remembers.
“If you read the worst-case scenarios, the children die within 12 months.
“In [Sofia’s] case, it’s a slow, neurodegenerative disease. Obviously, I didn’t know that, and I had no idea how long she was going to live for.
“The doctors told us there’s nothing you can do, and I think it’s probably the most devastating news for any parent to hear.”
‘It’s a ticking time bomb’
But the diagnosis spurred Dr Teng into action. At first, she raised money for research because at that point, in 2014, the disease had only just been defined.
But as Sophia’s condition began to deteriorate, to the point where she is now unable to walk or talk, Michelle realised she needed to do more.
“Anyone who’s lived with a loved one with Parkinson’s, Alzheimer’s or anything that’s degenerative will see that it’s a ticking time bomb,” she said.
“It’s a steady decline, and as the years go by, you see the people you love start losing the ability to do certain things.
“We raised something like close to £200,000 in a short space of time, which was extremely encouraging, but to really find a treatment, and to bring that treatment to clinic, will take five to 10 million, and I realised that was not going to cut the mustard.”
‘You have to be an optimist’
So in April 2021, Dr Teng set up SynaptixBio, a biotech firm based in Oxford with the sole aim of finding a treatment for TUBB4a.
The company recently signed a global licensing deal with the Children’s Hospital of Philadelphia – the world’s leading TUBB4a leukodystrophy centre – in the US to accelerate the research process.
It aims to launch clinical trials for a treatment in 2024.
Dr Teng said: “I feel like because we’ve set up this company, we’re finally in the driving seat in finding a treatment for [Sofia], within those timelines that we believe will be on time for her.
“And when you work in biotech, you have to be an optimist.”
‘A game-changer’
Research suggests there are 1,650 babies worldwide who are born with TUBB4a every year. In the UK, it is thought to affect between 60-90 newborns.
But scientists fear the number of cases could be higher still, due to many patients being misdiagnosed with other conditions, such as cerebral palsy.
Read more:
Better treatment promise for those with rare diseases
New drug offers lifeline to 1,600 women
Desperate parents ‘freezing’ to keep their disabled children ‘alive’
A varied combination of symptoms makes it incredibly difficult to spot. The only way to confirm the disease is by genome sequencing and an MRI scan.
Dr Dan Williams, SynaptixBio CEO and co-founder, called the treatment the company is developing a potential “game-changer”.
“One of the most horrible things is that a lot of these children don’t actually make it into late teenagers,” he said.
“Hopefully, by stopping the progression of this disease, it could offer them a better quality of life as well as an extension to their lives as well.”